Table Of Content
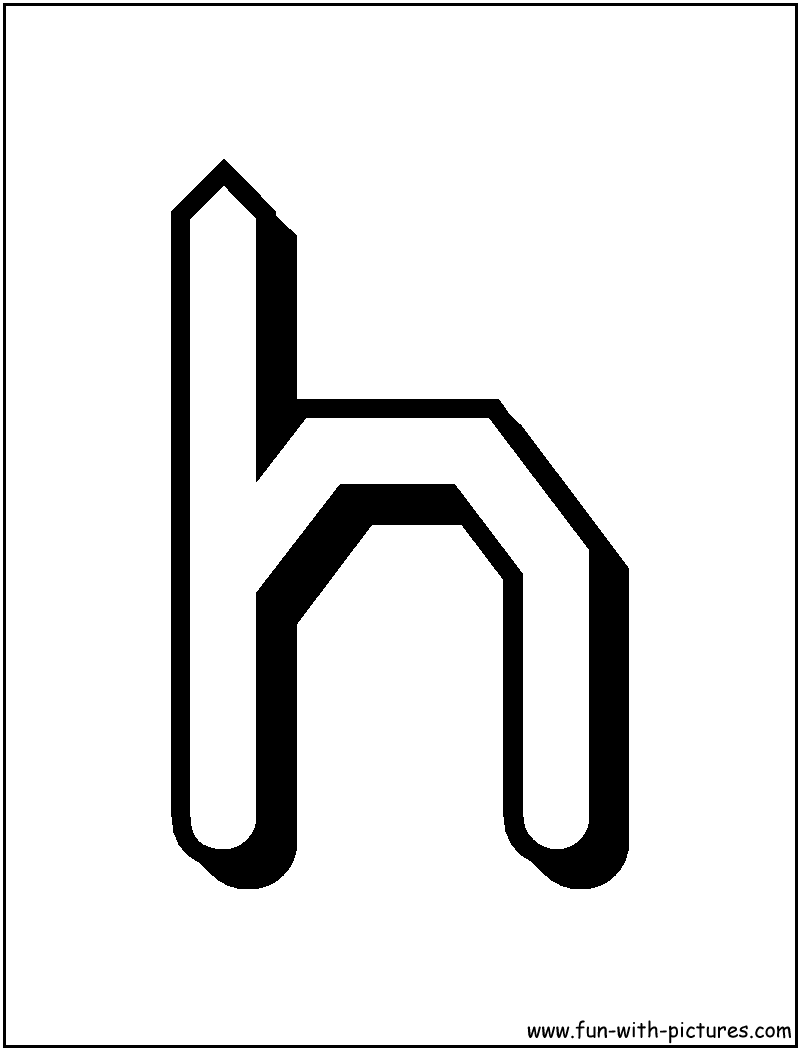
From Gaj's Latin alphabet h, from Czech alphabet h, from Latin h. Pronunciation as /xə/ is initial Slovene (phoneme plus a fill vowel) and the second pronunciation is probably taken from German h. The Finnish orthography using the Latin script was based on those of Swedish, German and Latin, and was first used in the mid-16th century. See the Wikipedia article on Finnish for more information, and h for information on the development of the glyph itself. The carbon may be sold as a manufacturing feedstock or fuel, or landfilled.

Derived terms
Consequently, kinetic energy is distributed faster through hydrogen than through any other gas; it has, for example, the greatest heat conductivity. Elementary hydrogen finds its principal industrial application in the manufacture of ammonia (a compound of hydrogen and nitrogen, NH3) and in the hydrogenation of carbon monoxide and organic compounds. Hydrogen is commonly used in power stations as a coolant in generators due to a number of favorable properties that are a direct result of its light diatomic molecules. These include low density, low viscosity, and the highest specific heat and thermal conductivity of all gases.
Related Words
See the history of Polish orthography article on Wikipedia for more, and h for development of the glyph itself. See the Kashubian alphabet article on Wikipedia for more, and h for development of the glyph itself. In Basque, during the 20th century it was not used in the orthography of the Basque dialects in Spain but it marked an aspiration in the North-Eastern dialects.
Descendants and related characters in the Latin alphabet
Hence hydrogen is often used as a theoretical model for more complex atoms, and the results are applied qualitatively to other atoms. These differ in the magnetic interactions of the protons due to the spinning motions of the protons. In ortho-hydrogen, the spins of both protons are aligned in the same direction—that is, they are parallel. In para-hydrogen, the spins are aligned in opposite directions and are therefore antiparallel. The relationship of spin alignments determines the magnetic properties of the atoms. Normally, transformations of one type into the other (i.e., conversions between ortho and para molecules) do not occur and ortho-hydrogen and para-hydrogen can be regarded as two distinct modifications of hydrogen.
Symbol
For example, in Italian h is used in combination with c or g to indicate the hard sound before a front vowel (e.g., chi, ghetto). The Greek Eta 'Η' in archaic Greek alphabets, before coming to represent a long vowel, /ɛː/, still represented a similar sound, the voiceless glottal fricative /h/. In this context, the letter eta is also known as Heta to underline this fact.
Compounds
Some languages, including Czech, Slovak, Hungarian, Finnish, and Estonian use ⟨h⟩ as a breathy voiced glottal fricative [ɦ], often as an allophone of otherwise voiceless /h/ in a voiced environment. Many metals such as zirconium undergo a similar reaction with water leading to the production of hydrogen. Hydrogen-lifted airships were used as observation platforms and bombers during the war. The letter H/h (like F/f, and O/o representing [o], [oː] instead of [uə̯]) is found only in words of foreign origin (borrowings).
Thermal and physical properties
It was accordingly put to a new use to indicate the open long e which had arisen through alteration of the primitive Greek long a. In a few inscriptions from Thera, Naxos, and several other localities the letter was used with syllabic value; that is, it included he, thus showing its old consonantal and its new vocalic value at the same time. Eventually, as a result of the spread of the Ionic alphabet, its use for the long vowel e or η became general throughout Greece, while its consonantal value as the aspirate h passed from the western Greek alphabets into the Etruscan alphabets and then into the Latin and other alphabets of ancient Italy. In the Romance languages the sound has largely disappeared, but the letter is still extensively used, partly with only etymological value, (e.g., French homme), partly with fancied etymological value (e.g., French haut from Latin altus, with h through the influence of hoh, the Old High German word of the same meaning), partly with special orthographical functions.
WMU CB Keni-H Lovely lands undrafted free agent deal with Buffalo Bills - MLive.com
WMU CB Keni-H Lovely lands undrafted free agent deal with Buffalo Bills.
Posted: Mon, 29 Apr 2024 12:11:00 GMT [source]
The gas, however, was confused with other flammable gases, such as hydrocarbons and carbon monoxide. In 1766 Henry Cavendish, English chemist and physicist, showed that hydrogen, then called flammable air, phlogiston, or the flammable principle, was distinct from other combustible gases because of its density and the amount of it that evolved from a given amount of acid and metal. In 1781 Cavendish confirmed previous observations that water was formed when hydrogen was burned, and Antoine-Laurent Lavoisier, the father of modern chemistry, coined the French word hydrogène from which the English form is derived. In 1929 Karl Friedrich Bonhoeffer, a German physical chemist, and Paul Harteck, an Austrian chemist, on the basis of earlier theoretical work, showed that ordinary hydrogen is a mixture of two kinds of molecules, ortho-hydrogen and para-hydrogen. Because of the simple structure of hydrogen, its properties can be theoretically calculated relatively easily.
Word History and Origins
In most dialects of Polish, both ⟨h⟩ and the digraph ⟨ch⟩ always represent /x/. In Ukrainian and Belarusian, when written in the Latin alphabet, ⟨h⟩ is also commonly used for /ɦ/, which is otherwise written with the Cyrillic letter ⟨г⟩. Steam reforming is also used for the industrial preparation of ammonia.
For example, in the solar wind they interact with the Earth's magnetosphere giving rise to Birkeland currents and the aurora. Phoenician and proto-Semitic languages are the earliest recorded alphabets that use symbols to represent sounds rather than to represent things like Egyptian hieroglyphics. (Greek is considered the first true alphabet because it uses symbols to represent both consonant and vowel sounds). In proto-Semitic, the letter H was also the word for thread or fence, and if you look at the letter H, it is still clear that it looks like a portion of a fence. The letter is silent in a syllable rime, as in ah, ohm, dahlia, cheetah, pooh-poohed, as well as in certain other words (mostly of French origin) such as hour, honest, herb (in American but not British English) and vehicle (in certain varieties of English). Initial /h/ is often not pronounced in the weak form of some function words including had, has, have, he, her, him, his, and in some varieties of English (including most regional dialects of England and Wales) it is often omitted in all words (see '⟨h⟩'-dropping).
Both of these forms result from writing the letter without taking the pen from the paper, the right-hand vertical bar being thus foreshortened and the horizontal stroke rounded. From these came the Carolingian form as well as the modern minuscule h. It consists of two protons and two electrons held together by electrostatic forces. Like atomic hydrogen, the assemblage can exist in a number of energy levels. Hydrogen is transparent to visible light, to infrared light, and to ultraviolet light to wavelengths below 1800 Å. Because its molecular weight is lower than that of any other gas, its molecules have a velocity higher than those of any other gas at a given temperature and it diffuses faster than any other gas.
In the quantum mechanical treatment, the electron in a ground state hydrogen atom has no angular momentum at all—illustrating how the "planetary orbit" differs from electron motion. In the early Greek alphabets a form with three horizontal bars and the simpler form H were both widely distributed. In Etruscan the prevailing form was similar to the early Greek form, and the same or a similar form occurs in very early Latin inscriptions, but the form H came into general use in Latin, either from the Chalcidic Greek alphabet of Cumae or from some other source. The cursive Latin form resembled a stylized version of the modern minuscule h, as did the uncial form.
Even though it is often said that there are more known compounds of carbon than of any other element, the fact is that, since hydrogen is contained in almost all carbon compounds and also forms a multitude of compounds with all other elements (except some of the noble gases), it is possible that hydrogen compounds are more numerous. Compounds of hydrogen are often called hydrides, a term that is used fairly loosely. The term "hydride" suggests that the H atom has acquired a negative or anionic character, denoted H−, and is used when hydrogen forms a compound with a more electropositive element. The existence of the hydride anion, suggested by Gilbert N. Lewis in 1916 for group 1 and 2 salt-like hydrides, was demonstrated by Moers in 1920 by the electrolysis of molten lithium hydride (LiH), producing a stoichiometric quantity of hydrogen at the anode.[50] For hydrides other than group 1 and 2 metals, the term is quite misleading, considering the low electronegativity of hydrogen.
This ion has also been observed in the upper atmosphere of the planet Jupiter. The ion is relatively stable in the environment of outer space due to the low temperature and density. H+3 is one of the most abundant ions in the universe, and it plays a notable role in the chemistry of the interstellar medium.[100] Neutral triatomic hydrogen H3 can exist only in an excited form and is unstable.[101] By contrast, the positive hydrogen molecular ion (H+2) is a rare molecule in the universe. H2 is unreactive compared to diatomic elements such as halogens or oxygen.
During the standardization of Basque in the 1970s, the compromise was reached that h would be accepted if it were the first consonant in a syllable. Hence, herri ("people") and etorri ("to come") were accepted instead of erri (Biscayan) and ethorri (Souletin). For the dialects lacking the aspiration, this meant a complication added to the standardized spelling.
The thermodynamic basis of this low reactivity is the very strong H–H bond, with a bond dissociation energy of 435.7 kJ/mol.[26] The kinetic basis of the low reactivity is the nonpolar nature of H2 and its weak polarizability. It spontaneously reacts with chlorine and fluorine to form hydrogen chloride and hydrogen fluoride, respectively.[27] The reactivity of H2 is strongly affected by the presence of metal catalysts. Thus, while mixtures of H2 with O2 or air combust readily when heated to at least 500 °C by a spark or flame, they do not react at room temperature in the absence of a catalyst.

No comments:
Post a Comment